Each month we will highlight a different PSI plasmid or plasmid collection which may interest you.
Cleavable C-terminal His-tag vectors for structure determination from the MCSG
- Who: the Midwest Center for Structural Genomics
- What: LIC-compatible C-terminal His tagged vectors
- Why: To improve crystallization of proteins that failed in the crystallization pipeline with N-terminal tags
- Where: pMCSG26, pMCSG28, pMCSG29, and pMCSG32
In high-throughput structural biology centers that clone, express, purify and crystallize thousands of proteins at one time, salvage pathways must be used to attempt to capture proteins that failed within the pipeline.; The ability to purify and crystallize a protein for structure determination depends on numerous factors; therefore numerous salvage pathway methods have been developed. 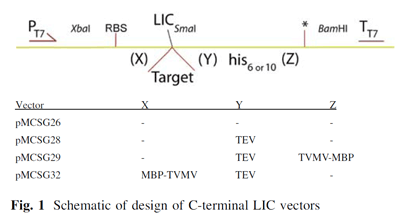 One method developed by the Midwest Center for Structural Genomics (MCSG) re-engineers their standard vector (pMCSG7) appending 6xHis-tag to N-terminus of protein to create a ligation independent cloning (LIC) compatible vector (pMCSG26) appending 6xHis tag to C-terminus. Variations of this vector add a TEV protease cleavage site to remove the His tag from the expression protein (pMCSG28) or an additional MBP tag that can be removed by TVMV protease (pMCSG29 and pMCSG32) (Figure 1). More details can be found on the MCSG website.
One method developed by the Midwest Center for Structural Genomics (MCSG) re-engineers their standard vector (pMCSG7) appending 6xHis-tag to N-terminus of protein to create a ligation independent cloning (LIC) compatible vector (pMCSG26) appending 6xHis tag to C-terminus. Variations of this vector add a TEV protease cleavage site to remove the His tag from the expression protein (pMCSG28) or an additional MBP tag that can be removed by TVMV protease (pMCSG29 and pMCSG32) (Figure 1). More details can be found on the MCSG website.
The MCSG tested these vectors with 16 proteins that were soluble in the N-terminal vector but failed at various later stages in the pipeline (protein would not bind to IMAC column, TEV protease would not cleave off His tag, protein fail to crystallize). In the C-terminal vectors (Eschenfeldt et al 2010), 10 of the expressed proteins crystallized and five led to structures (see example, Pfleger et al. 2008). Several mechanisms have been proposed to explain why C-terminal tags make a difference. The N-terminus may be buried and a N-terminal tag may affect protein folding, it may be involved in protein oligomerization or disrupt protein packing in the crystal, whereas if the C-terminal tag is open to solvent it would not. Also C-terminal tags ensure that only the full-length protein is purified by affinity chromatography and avoids the possible defects in crystallization by proteins truncated by premature termination. Whatever the reason, these C-terminal vectors clearly provide a viable method to salvage proteins that do not crystallize with N-terminal appendage.
References
Eschenfeldt, W.H., Maltseva, N., Stols, L., Donnelly, M.I., Gu, M., Nocek, B., Tan, K., Kim, Y. , and Joachimiak, A. (2010) Cleavable C-terminal His-tag vectors for structure determination. Journal of Structural and Functional Genomics 11:31-39. PMID:20213425
Pfleger, B.F., Kim, Y., Nusca, T.D., Maltseva, N., Lee, J. Y., Rath, C.M., Scaglione, J.B., Janes, B.K., Anderson, E.C., Bergman, N.H., Hanna, P.C., Joachimiak, A., and Sherman, D.H. (2008) Structural and functional analysis of AsbF: Origin of the stealth 3,4-dihydroxybenzoic acid subunit for petrobactin biosynthesis. PNAS 105 (44):17133-17138. PMID:18955706
Updated for March 2011
Click here to see an archive of previous Plasmid of the Month features.

