Each month we will highlight a different PSI plasmid or plasmid collection which may interest you.
Vectors for Wheat Germ Cell-Free Protein Expression
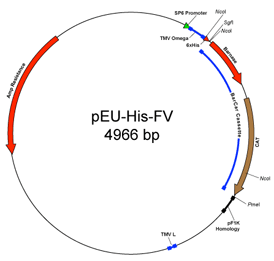 The University of Wisconsin Center for Eukaryotic Structural Genomics (CESG) developed the plasmids pEU-His-FV and pEU-HSBC for use in wheat germ cell-free protein synthesis. They are derived from a vector originally created by Prof. Yaeta Endo at the Cell-Free Science and Technology Research Center, Ehime University, Japan (1). The wheat germ cell-free translation vectors contain the SP6 promoter to facilitate in vitro transcription of the inserted gene and an engineered translational enhancer omega sequence derived from tobacco mosaic virus to promote efficient initiation of eukaryotic translation without a 5’ capping structure.
The University of Wisconsin Center for Eukaryotic Structural Genomics (CESG) developed the plasmids pEU-His-FV and pEU-HSBC for use in wheat germ cell-free protein synthesis. They are derived from a vector originally created by Prof. Yaeta Endo at the Cell-Free Science and Technology Research Center, Ehime University, Japan (1). The wheat germ cell-free translation vectors contain the SP6 promoter to facilitate in vitro transcription of the inserted gene and an engineered translational enhancer omega sequence derived from tobacco mosaic virus to promote efficient initiation of eukaryotic translation without a 5’ capping structure.
CESG modified the parent vector to be compatible with the Promega Flexi® Vector system, which allows directional cloning into SgfI and PmeI restriction sites. Strong positive selection for cloning is provided by substitution of the ORF of interest for a lethal gene (2, 3). In pEU-His-FV, the highly toxic barnase gene provides the lethal selection. In order to propagate vectors containing the barnase gene, Escherichia coli strains containing the detoxifying barstar gene must be used (e.g. BR610). Currently, Promega (Madison, WI) provides these strains by request, and DNASU distributes pEU-His-FV as a glycerol stock in this strain. However, the toxicity of the barnase gene complicated our routine use of these plasmids. In order to address this technical issue, we created pEU-HSBC by substitution of sacB for barnase. The sacB gene encodes levansucrase, a secreted enzyme that hydrolyzes sucrose to glucose and fructose. The fructosyl units are then polymerized to form levan (4). Expression of sacB confers sensitivity to sucrose in many bacterial species and has been used extensively as a negative selectable marker (5). Unlike pEU-His-FV, pEU-HSBC can be propagated in any E. coli strain (and is distributed by DNASU in phage resistant DH5-alhpa bacteria), but maximal cloning efficiency is obtained by propagation in dam– strains. When pEU-HSBC is used for cloning, a medium supplemented with ampicillin and 5% sucrose must be used to obtain selection against non-recombinant transformants retaining the sacB gene. At CESG, the standard practice is to sequence-verify all cloned genes before either cell-free translation experiments or transfer of the gene into different FlexiVector plasmids (also available from the PSI-MR).
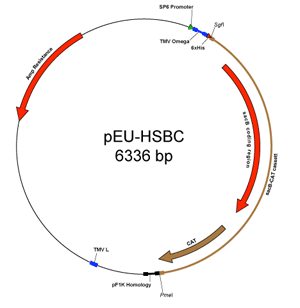 The vectors highlighted here do not encode a protease cleavage site for removal of the His-tag. Instead, CESG had developed a two-step PCR protocol to add the 5’ nucleotide sequence encoding a TEV protease cleavage site along with the required 5’ Sgf I and 3’ Pme I restriction sites during amplification of the ORF (3). The PCR product is cloned into FlexiVector-compatible vectors using a FlexiVector ORF capture protocol or Promega’s suggested method.
The vectors highlighted here do not encode a protease cleavage site for removal of the His-tag. Instead, CESG had developed a two-step PCR protocol to add the 5’ nucleotide sequence encoding a TEV protease cleavage site along with the required 5’ Sgf I and 3’ Pme I restriction sites during amplification of the ORF (3). The PCR product is cloned into FlexiVector-compatible vectors using a FlexiVector ORF capture protocol or Promega’s suggested method.
The CESG cloning approach supports proteolytic release of the His6-tag from a translated protein, yielding Ser as the first residue of the mature protein followed by the second residue of the ORF. Methods for use of pEU-His-FV and pEU-HSBC in cell-free translation of both soluble and membrane proteins have been published (6, 7) and are available from the PSI-SBKB along with specific examples of their use with many different proteins.
Russell L. Wrobel, John G. Primm, and Brian G. Fox
Publications
- Sawasaki, T., Ogasawara, T., Morishita, R., and Endo, Y. (2002) A cell-free protein synthesis system for high-throughput proteomics, Proc Natl Acad Sci U S A 99, 14652-14657.
- Blommel, P. G., Martin, P. A., Wrobel, R. L., Steffen, E., and Fox, B. G. (2006) High efficiency single step production of expression plasmids from cDNA clones using the Flexi Vector cloning system, Protein Expr Purif 47, 562-570.
- Blommel, P. G., Martin, P. A., Seder, K. D., Wrobel, R. L., and Fox, B. G. (2007) Flexi Vector cloning in high throughput protein expression and purification, in Methods in Molecular Biology (Doyle, S., Ed.), The Humana Press Inc., Totowa, N.J.
- Steinmetz, M., Le Coq, D., Djemia, H. B., and Gay, P. (1983) [Genetic analysis of sacB, the structural gene of a secreted enzyme, levansucrase of Bacillus subtilis Marburg], Mol Gen Genet 191, 138-144.
- Gay, P., Le Coq, D., Steinmetz, M., Berkelman, T., and Kado, C. I. (1985) Positive selection procedure for entrapment of insertion sequence elements in gram-negative bacteria, J Bacteriol 164, 918-921.
- Makino, S.-I., Goren, M. A., Fox, B. G., and Markley, J. L. (2009) Cell-free protein synthesis technology in NMR high-throughput structure determination, Methods in Molecular Biology 2010.
- Goren, M., Nozawa, A., Makino, S., Wrobel, R., and Fox, B. (2009) Cell-free translation of integral membrane proteins into unilamelar liposomes, Methods Enzymol 463, 647-673.
Updated for August 2010
Click here to see an archive of previous Plasmid of the Month features.

